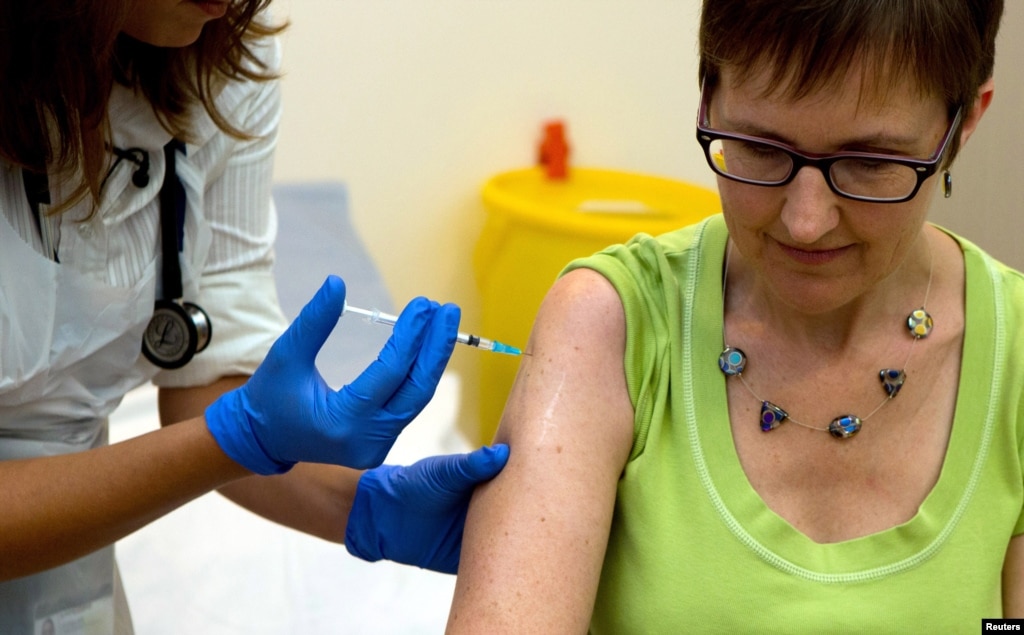
Researchers are hurrying to develop effective treatments and vaccines for Ebola. The disease has killed more than 4,500 people. Most of the victims lived in one of three West African countries: Guinea, Liberia or Sierra Leone.
In the United States, the government’s process for approving new drugs can often take years. But treatments for Ebola are moving quickly through the approval process.
Before US government officials approve a new drug, researchers must perform detailed scientific tests -- what are called clinical trials. The tests must be done on human beings to show that the experimental treatment is both safe and effective.
These drug trials usually involve thousands of people and can take many years to complete. But there are exceptions.
Thomas Geisbert is a microbiologist at the University of Texas Medical Branch in Galveston. He says the US Food and Drug Administration can make an exception through what he calls its “animal rule.” This enables researchers to get faster approval when faced with a deadly disease like the Ebola virus.
Mr. Geisbert helped develop what is known as the VSV Ebola vaccine. This vaccine recently moved to human clinical trials at the Walter Reed Army Institute of Research in Maryland. The tests involve 20 healthy volunteers.
Mr. Geisbert says that the “animal rule” requires only that scientists demonstrate that a treatment is effective in an animal model of human disease. This time, he says, the human model of disease was monkeys.
“And then in conjunction with that, you do a conventional phase one trial. That’s just a study where you put a vaccine or treatment into a healthy human volunteer just to make sure that you know in normal healthy people that your vaccine or drug does not cause any disease or serious adverse event.”

Another experimental drug, called zMapp, went straight from the laboratory to Ebola patients. The Food and Drug Administration also bypassed human safety trials to treat a handful of patients in the United States.
Many researchers are hard at work to develop effective treatments and vaccines for Ebola. Canadian researchers made the VSV vaccine, which is now being tested in Maryland.
The British drug company GlaxoSmithKline has made another promising, but experimental vaccine. The safety of that vaccine is currently being tested in Mali. The West African nation shares a border with Guinea, where the virus has infected many people. As of now, no Ebola cases have been reported in Mali.
Researchers say the promising vaccine will move into a more advanced human testing early next year. They will use these tests to show if the vaccine is safe for human use and if it is effective in preventing Ebola.
Mike Levine is director of the Center for Vaccine Development at the University of Maryland’s School of Medicine. He is helping to direct human studies of the experimental vaccine in Mali.
Mr. Levine says the second part of testing will involve health care workers. He says protecting those closest to Ebola patients will help control the spread.
“If the vaccine is working in humans, like it worked in non-human primates (monkeys) then we may be able to greatly diminish transmission - maybe even interrupt transmission - in localized areas by immunizing contacts of known patients and doing a very good job of immunizing health care workers.”
Both vaccines proved to be 100 percent effective in protecting monkeys against infection with the Ebola virus. And they produced no side effects.
But as for Ebola treatments, Thomas Geisbert says that it is too early to know if what works in animals will also work in humans.
“I mean we don’t know if any of these vaccines is going to work in humans, quite honestly.”
Health officials hope to have early results of the VSV vaccine trials in Maryland by December. They plan to move to clinical safety trials in Africa shortly after that -- most likely in health care workers treating Ebola patients. Health care workers are at greatest risk of infection, say public health officials.
Mr. Geisbert says the vaccines, especially the VSV, not only have the potential to prevent Ebola. He says they also could treat the disease soon after a person is infected.
I’m Anna Matteo.
*This report was based on stories from VOA reporter Jessica Berman. Anna Matteo adapted it for Learning English. George Grow was the editor.
Words in this Story
clinical – adj. relating to or based on work done with real patients
trial – n. one of a number of repetitions of an experiment
microbiology – n. a science that studies extremely small forms of life (such as bacteria and viruses). A microbiologist studies microbiology.
Now it’s your turn to use these Words in this story. In the comments section, write a sentence using one of these words and we will provide feedback on your use of vocabulary and grammar.
Or simply share your opinion about this story.


